


|
|||
 |
| ||
Intermolecular Forces | |||
Classifying intermolecular forces (part 2).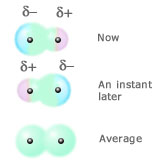
Some molecules have a permanent dipole moment - averaged over time there is an unevenness or lack of symmetry in the distribution of charge in the molecule. One end of the molecule has a partial positive charge compared to the other end of the molecule. Even if a molecule doesn't have a permanent dipole moment, at any instant in time it will almost certainly have a transient dipole moment (or temporary dipole moment) because the distribution of electrons will not be symmetric enough to produce a zero dipole moment. Over time these transient dipole moments will average out to zero, but at any instant they can give rise to intermolecular forces between nearby molecules. A molecule with zero permanent dipole moment can acquire a dipole moment if it is placed in an electric field. The field distorts the distribution of charge in the molecule producing an induced dipole moment. Any molecule with a dipole moment (either permanent or transient) produces an electric field and so can induce a dipole moment in neighbouring molecules. These induced dipole moments will always be oriented to cause the molecules to attract each other. The size of the induced dipole moment will depend on how easy it is to distort the electron cloud in the molecule. This is called the polarizability of the molecule. Polarizability depends on how large the atoms making up a molecule are (for example, bromine is a larger atom than chlorine - see WebElements) and the number of atoms in the molecule. The larger an atom is, the less tightly the outer electrons are held and the more easily the electron cloud can be distorted by an electric field. The attraction between a transient dipole moment in one molecule and the induced dipole in another molecule is called a dispersion force.
|