


|
|||
 |
| ||
Intermolecular Forces | |||
Classifying intermolecular forces.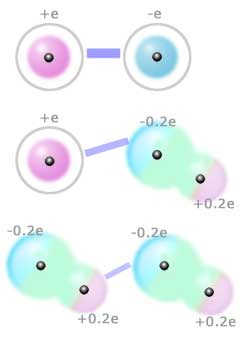
Since intermolecular forces originate in the attractions and repulsions between charges, to explain the relative strengths of these forces it would be sensible to classify molecules on the basis of the charge they carry or the distribution of charges within them. An ion (like Na+ or Br-) has a charge of at least ±e, where e is the charge on an electron. The force between a pair of oppositely charged ions will be strong - it is an ionic bond, stronger than the forces we are considering here. An ion will also exert a force on a neutral (no overall charge) molecule, but the force will be much weaker than an ionic bond because a neutral molecule does not have a net charge, although there may be partial charges on some parts of the molecule (for example, it may have a dipole moment). The partial charges that give rise to a molecular dipole moment are usually much less than the full charge on an electron (e). For example if you calculate the charges on the H and Cl atoms required to produce the observed dipole moment of a hydrogen chloride (HCl) molecule, these charges are only +0.2e (on the hydrogen atom) and -0.2e (on the chlorine atom). Thus the force between an ion and a polar molecule is much weaker than an ionic bond because the ion is being attracted or repelled by a much smaller charge than on another ion. The figure on the left represents the forces between a pair of ions, an ion and a dipolar molecule, and a pair of polar molecules. Similarly the force between a pair of molecules each with a dipole moment is weaker than between an ion and a polar molecule. The strength of the force between a pair of polar molecules also decreases more rapidly with increasing distance than the force between a pair of ions. When interpreting the strength of intermolecular forces you need to note whether the molecules have overall charges (that is, they are ions) or permanent dipole moments. But this isn't the whole story...
|