


|
|||
 |
| ||
Intermolecular Forces | |||
Dipole-induced dipole forces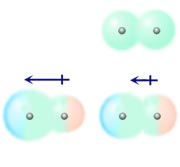
A polar molecule produces an electric field which can distort the electron cloud of a nearby molecule. If a non-polar molecule (top right of the adjacent picture) is brought near a polar molecule (lower left) the field from the polar molecule can induce a dipole moment in a nearby non-polar molecule (lower right). This will cause the an attraction between the molecules. This type of force is responsible for the solubility of oxygen (a non-polar molecule) in water (polar). The size of the induced dipole depends on the ease with which the electron distribution in the non-polar molecule can be distorted by the electric field of the polar molecule. This is a property of any molecule called its polarizability. In general molecular polarisability is larger for molecules with a larger volume (for example, molecules containing atoms with large atomic number).
|