


|
|||
 |
| ||
Intermolecular Forces | |||
What causes intermolecular forces?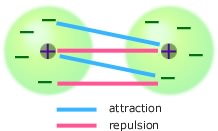
Molecules are made up of charged particles: positive nuclei and negative electrons. When one molecule approaches another there is a multitude of forces between the particles in the two molecules. Each electron in one molecule is attracted to the nuclei in the other molecule but also repelled by the electrons in the other molecule. The nuclei in one molecule are repelled by the nuclei in the other molecule, but also attracted by the electrons in the other molecule. In principle you could calculate (at least approximately) all the forces and add them up to get the total force between the two molecules. This can be done for an increasingly large range of molecules, but for many problems such detailed calculations cannot be justified in terms of time nor cost. A simpler approach is to find molecular properties which determine the strength of the intermolecular forces and to do this we need to classify the interactions between the molecules into a few broad categories based on particular properties of the molecules we are considering.
|