


|
|||
 |
| ||
Intermolecular Forces | |||
Dipole moments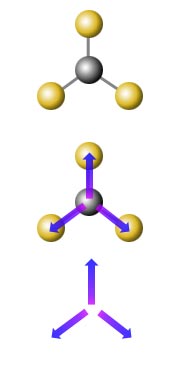
The dipole moment is a measure of the unevenness, or lack of symmetry, of the charge distribution in a molecule. The mathematical definition of the dipole moment involves adding up the size of each charge in the molecule multiplied by the average distance that charge is from an arbitrary origin. Any molecule where the overall centre of the positive charges and the overall centre of the negative charges coincide will have zero dipole moment. This happens in molecules with sufficiently symmetric shape. So it is the shape of a molecule that is important in determining whether it has a dipole moment. A dipole moment is a vector quantity - it has both size and direction. You can think of each chemical bond in a molecule as having a dipole moment oriented parallel to the bond, with a size and direction determined by the atoms at each end of the bond. These bond dipole moments will be large if the bonded atoms have very different electronegativities (see this WebElements page for a graph of electronegativity across the periodic table). For example, in the molecule BF3, each BF bond has a dipole moment with a partial negative charge on the fluorine (it is much more electronegative than the boron), but the shape of the molecule (trigonal planar) is such that the bond dipole moments add up to zero. To determine if a molecule has a permanent dipole moment (and hence experiences dipole-dipole forces) you need to be able to draw a Lewis electron-dot structure for the molecule and apply VSEPR theory to determine the shape of the molecule.
|