


|
|||
 |
| ||
Intermolecular Forces | |||
Dispersion forces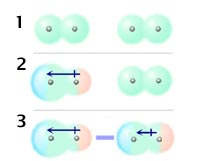
Dispersion forces (or London forces, after the scientist Fritz London) arise from the temporary variations in electron density around atoms and molecules. The average shape and electron distribution for non-polar molecules have a certain minimum symmetry or eveness. Figure 1 on the left depicts the average electron clouds of two nonpolar molecules. However at any instant the electron distribution around an atom or molecule will likely produce a dipole moment (figure 2) which will average out to zero over a period of time. But even a temporary or transient dipole moment can induce a (temporary) dipole moment in any nearby molecules (picture 3) causing them to be attracted to the first molecule. It is the polarisability of the molecules which determines the size of the temporary and induced dipole moments and thus the strength of the dispersion forces. Molecules containing large atoms (e.g. bromine or iodine) have large polarisabilities and so give rise to large dispersion forces. This explains the increasing melting and boiling points of the halogens going down that group of the periodic table. Dispersion forces are often the strongest intermolecular forces acting between molecules even though they originate in temporary dipoles and induced dipole moments. Unlike forces between molecules with permanent dipole moments, dispersion forces always act to attract the molecules to each other regardless of the relative orientation of the molecules (except once the molecules get very close, when all types of intermolecular forces become repulsive).
|