


|
|||
 |
| ||
Intermolecular Forces | |||
Intermolecular forces - attraction and repulsion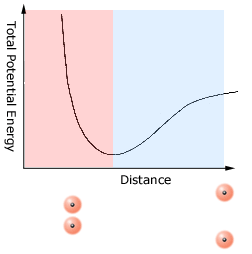
People usually think of intermolecular forces as being only attractive (pulling molecules towards each other, not pushing them apart). Perhaps this is because such forces do attract molecules to each other over a wide range of distances. But once a pair of molecules get very close, the intermolecular forces try to push them apart. The usual way to depict the effect of intermolecular forces is to draw a graph showing the total (potential) energy of a pair of molecules as the distance between the molecules is varied. As the molecules get closer the forces between the charged particles in each molecule result in a lowering of the total energy until a minimum value is reached. In this region (shaded blue) the intermolecular forces are attractive - if the molecules move closer together the total energy goes down. But once the minimum is reached any further reduction in the distance between the molecules causes the potential energy to increase (red region). In this region the intermolecular forces are repulsive. If intermolecular forces did not repel molecules at short distances all molecules would simply coalesce. Another way to think about what happens when two molecules get very close is to picture what happens at very small separations. The two positively charged nuclei almost sit on top of each other as do the negatively charged electron clouds. It is this close approach of the like-charged nuclei (and electrons) which produces the replusion between the molecules at small separations. (Note: the size and direction of the intermolecular force is related to the slope or derivative of this curve. The sign of that derivative changes at the minimum in the curve.)
|