


|
|||
 |
| ||
Intermolecular Forces | |||
Dipole - dipole forces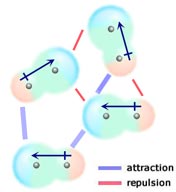
If two neutral molecules, each having a permanent dipole moment, come together such that their oppositely charged ends align, they will be attracted to each other. In a liquid or solid these alignments are favoured over those where like-charged ends of the molecules are close together and hence repel each other. One reason why CH3F has a higher boiling point (-84 °C) than CF4 (-128 °C) is that CF3H has a permanent dipole moment, while CF4 does not. To fully understand this you need to know that CF4 is tetrahedral in shape and CH3F is roughly tetrahedral (there is not a big difference in the shapes of these molecules). The carbon - fluorine bonds in both molecules are polar (fluorine is much more electronegative than carbon) however the presence of four C-F bonds in the tetrahedral CF4 molecule makes the molecule overall non-polar. The dipole moment of the single C-F bond in CH3F is not cancelled out by the C-H bond dipoles (which are very small) so CH3F does have a permanent dipole moment. A type of intermolecular force which can arise in particular circumstances and is usually classified as a dipole-dipole interaction, although it has some covalent bond character is hydrogen bonding.
|