


|
|||
 |
| ||
Intermolecular Forces | |||
Hydrogen bonding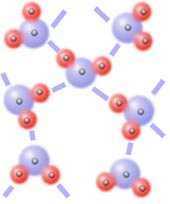
Hydrogen bonds are usually listed as a type of dipole-dipole force, but the details of hydrogen bonding are subtle and these bonds have some partial covalent bond character. Hydrogen bonds form only between a limited number of elements bonded in a specific sequence. If -A-H is part of one molecule and B- is part of a second molecule (or another portion of the first molecule), a hydrogen bond will form only if A and B are small, electronegative atoms (either N, O, or F). The hydrogen bond involves a lone pair of electrons on atom B being attracted to the hydrogen atom which has had some of its electron cloud pulled from it by atom A.
So a necessary condition for hydrogen bonding is that one molecule must contain a H bonded to either N, O, or F; and the other molecule must contain either N, O, or F. If a hydrogen bond can form between a pair of molecules it will be stronger than other intermolecular forces between the molecules. Hydrogen bonding is responsible for the unexpectedly high boiling point of water (compared to the other group 16 hydrides such as H2S, H2Se etc.), the lower density of ice compared to liquid water (see picture on left), and the shape of many essential biological molecules (e.g. DNA and proteins).
|