


|
|||
 |
| ||
Intermolecular Forces | |||
How strong are intermolecular forces?One measure of the strength of a chemical bond is the energy required to break one of those bonds. This will be a very small quantity so it is usual to quote bond strength as the energy required to break one mole of bonds. This is equal to the energy required to break one bond multiplied by the Avogadro constant. Intermolecular forces are weaker than the forces we call chemical bonds. Typical strengths for chemical bonds (ionic, covalent, metallic) are in the range of hundreds or thousands of kilojoules per mole. Intermolecular forces are usually less than 50 kJ/mol. One problem with using the words 'weak' and 'strong' to describe intermolecular forces is that for a particular pair of molecules the force between then will always be weak when they are a long way apart and there will always be a strong force repelling them once they get close together. So what are we really saying when we say the intermolecular forces between particular molecules are weak or strong? To answer this look at graphs of how the total (potential) energy of a pair of molecules varies as the distance between them changes. 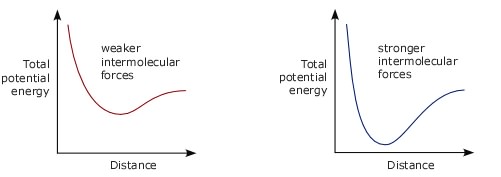
If a pair of molecules have strong intermolecular forces between them what we are saying is that the total energy of the molecules decreases by a large amount around the distance where the force changes from attracting the molecules to each other to repelling them. The drop in energy is not so large if the intermolecular forces are weak.
|