


|
|||
 |
| ||
Intermolecular Forces | |||
Review -1For each of the molecules below, list the types of intermolecular force which act between pairs of these molecules. (a) CH4, (b) PF3, (c) CO2, (d) HCN, (e) HCOOH (methanoic acid) HintsDispersion forces act between all molecules. AnswersTo determine the types of intermolecular force between molecules you first have to determine if the molecules are polar, and this means you need to know the shape of the molecule. The diagrams below show the shapes of these molecules. 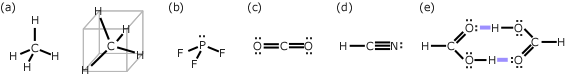
(a) CH4 is a tetrahedral molecule - it does not have a permanent dipole moment. The figure above shown CH4 in two views: one shows it as it is commonly drawn, with one H at the top and three H's at the bottom. The second figure shows CH4 rotated to fit inside a cube. This might help to make clear why it does not have a permanent dipole moment. The dipole moments of the two C-H bonds pointing up exactly cancel the dipole moments of the two C-H bonds pointing downward. CH4 does not contain N, O, or F and therefore there are no hydrogen bonds between CH4 molecules. Therefore only dispersion forces act between pairs of CH4 molecules. Other tetrahedral molecules (like CF4, CCl4 etc) also do not have a permanent dipole moment. (b) PF3 is a trigonal pyramidal molecule (like ammonia, the P has a single lone pair of electrons); it does have a permanent dipole moment. It does contain F, but it does not contain any hydrogen atoms so there is no possibility of forming hydrogen bonds. Therefore dispersion forces and dipole-dipole forces act between pairs of PF3 molecules. (c) CO2 is a linear molecule; it does not have a permanent dipole moment; it does contain O, however the oxygen is not bonded to a hydrogen. Therefore only dispersion forces act between pairs of CO2 molecules. (d) HCN is a linear molecule; it does have a permanent dipole moment; it does contain N, however the nitrogen is not directly bonded to a hydrogen. Therefore dispersion forces and dipole-dipole forces act between pairs of HCN molecules. (e) HCOOH is a non-linear molecule; it does have a permanent dipole moment; it does contain O, and the oxygen is directly bonded to a hydrogen. Therefore dispersion forces, dipole-dipole forces and hydrogen bonds act between pairs of HCOOH molecules. Because hydrogen bonds are considered as a type of dipole-dipole force, some books will just list dispersion forces and hydrogen bonds as relevant to methanoic acid. The picture above shows a pair of HCOOH molecules (a dimer) joined by a pair of hydrogen bonds. Other organic (carboxylic) acids such as acetic acid form similar dimers.
|